Principles of working of a laser
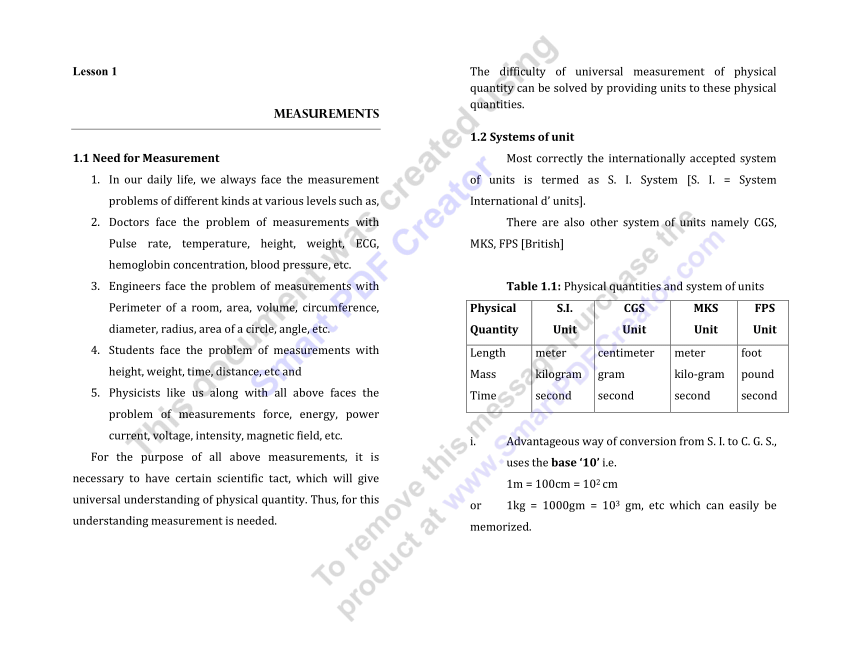
Output Characteristics of the Laser. Shimoda, Professor Koichi. Introduction to Laser Spectroscopy is strongly recommended to newcomers as well as researchers in physics, engineering, chemistry and biology. Show less Introduction to Laser Spectroscopy is a well-written, easy-to-read guide to understanding the fundamentals of lasers, experimental methods of modern laser spectroscopy and applications. 1 CHAPTER 1 Introduction to Physics Physics (from the Greek, φυσικός (phusikos), 'natural', and φύσις (phusis), 'nature') is the science of Nature in the broadest sense. Physicists study the behavior and properties of matter in a wide variety of contexts, ranging from the sub-nuclear particles from which all ordinary matter is made (particle physics) to the behavior of the material. Home / Learn / Microscopy Resource Center / Microscopy Primer / The Physics of Light and Color / Laser Fundamentals - Introduction to Lasers In popular science fiction videos during the 1950s, monsters were often portrayed that could emit lethal rays of light from their eyes (Figure 1), but until the invention of the laser, such concentrated.
Introduction To Laser Physics Pdf
In lasers, photons are interacted in three ways with the atoms:
- Absorption of radiation
- Spontaneous emission
- Stimulated emission
Absorption of radiation
Absorptionof radiation is the process by which electrons in the ground state absorbs energy from photons to jump into the higher energy level.
Theelectrons orbiting very close to the nucleus are at the lower energy level or lower energy state whereas the electrons orbiting farther away from the nucleus are at the higher energy level. The electrons in the lower energy level need some extra energy to jump into the higher energy level. This extra energy is provided from various energy sources such as heat, electric field, or light.
Let us consider two energy levels (E1 and E2) of electrons. E1 is the ground state or lower energy state of electrons and E2 is the excited state or higher energy state of electrons. The electrons in the ground state are called lower energy electrons or ground state electrons whereas the electrons in the excited state are called higher energy electrons or excited electrons.
In general, the electrons in the lower energy state can’t jump into the higher energy state. They need sufficient energy in order jump into the higher energy state.
When photons or light energy equal to the energy difference of the two energy levels (E2 – E1) is incident on the atom, the ground state electrons gains sufficient energy and jumps from ground state (E1) to the excited state (E2).
Theabsorption of radiation or light occurs only if the energy of incident photon exactly matches the energy difference of the two energy levels (E2 – E1).
Spontaneous emission
Spontaneousemission is the process by which electrons in the excited state return to the ground state by emitting photons.
Theelectrons in the excited state can stay only for a short period. The time up to which an excited electron can stay at higher energy state (E2) is known as the lifetime of excited electrons. The lifetime of electrons in excited state is 10-8 second.
Introduction To Laser Physics Shimoda Pdf
Thus, after the short lifetime of the excited electrons, they return to the lower energy state or ground state by releasing energy in the form of photons.
Inspontaneous emission, the electrons move naturally or spontaneously from one state (higher energy state) to another state (lower energy state) so the emission of photons also occurs naturally. Therefore, we have no control over when an excited electron is going to lose energy in the form of light.
The photons emitted in spontaneous emission process constitute ordinary incoherent light. Incoherent light is a beam of photons with frequent and random changes of phase between them. In other words, the photons emitted in the spontaneous emission process do not flow exactly in the same direction of incident photons.
Stimulated emission

Stimulatedemission is the process by which incident photon interacts with the excited electron and forces it to return to the ground state.
Instimulated emission, the light energy is supplied directly to the excited electron instead of supplying light energy to the ground state electrons.
Unlike the spontaneous emission, the stimulated emission is not a natural process it is an artificial process.
Inspontaneous emission, the electrons in the excited state will remain there until its lifetime is over. After completing their lifetime, they return to the ground state by releasing energy in the form of light.
However, in stimulated emission, the electrons in the excited state need not wait for completion of their lifetime. An alternative technique is used to forcefully return the excited electron to ground state before completion of their lifetime. This technique is known as the stimulated emission.
Whenincident photon interacts with the excited electron, it forces the excited electron to return to the ground state. This excited electron release energy in the form of light while falling to the ground state.
In stimulated emission, two photons are emitted (one additional photon is emitted), one is due to the incident photon and another one is due to the energy release of excited electron. Thus, two photons are emitted.
Thestimulated emission process is very fast compared to the spontaneous emission process.
All the emitted photons in stimulated emission have the same energy, same frequency and are in phase. Therefore, all photons in the stimulated emission travel in the same direction.
The number of photons emitted in the stimulated emission depends on the number of electrons in the higher energy level or excited state and the incident light intensity.
It can be written as:
Number of emitted photons α Number of electrons in the excited state + incident light intensity.
